No-touch continuous monitoring


Why continuous monitoring
- Early warning of contamination threats
- Minimise process downtime
- Save time and resource by replacing manual sampling processes
- Demonstrate best practice in production Quality Assurance
- Maintain required conditions between ISO 14644-1 inspections

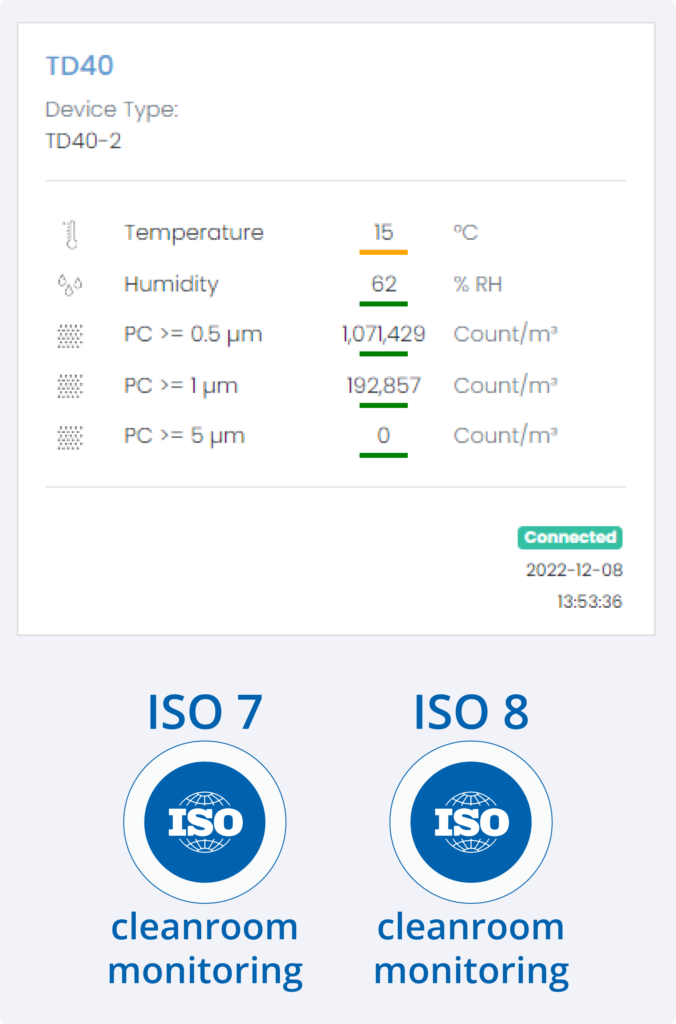
How it works
- High-precision sensors provide 20-60 second sampling of particle counts and related metrics
- Live status is verified using a simple Red / Amber / Green display on the HEX dashboard
- In the event of a particle spike an alarm is generated to enable immediate intervention
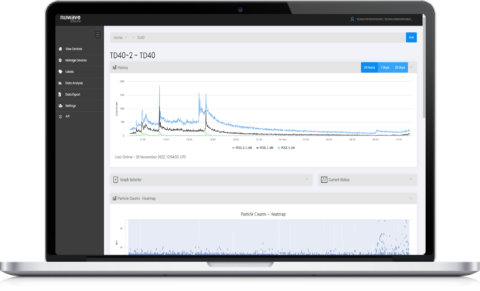
Remote Management & Particle Diagnostics
The HEX software platform is used for monitoring live particle status at a glance, across multiple devices and sites. The system can be left to run in the background with automated alerts to ensure that contamination threats can be responded to immediately.
Particles are categorised into 24 software bins ranging from 0.35μm to 40μm in size for detailed analysis of particle distribution, concentration and density. This enables analysis of cleanroom performance drift between ISO 14644-1 inspections.
Analytics tools help characterise the nature of particle spikes or trends. Data can be viewed holistically across all 24 bins with options to drill down to specific particle sizes. Data can be viewed live or historically and exported to csv if required for further analysis.
Ease of Operation & Integration
The plug and play system is quick to set up and maintenance-free operation ensures that personnel can rely on seamless background monitoring 24-7.
Users can view data output remotely on the HEX dashboard as well as on the customer’s own SCADA or preferred application.
Sensors are small, unobtrusive and portable should redeployment be required.
There are no regional-specific requirements and the system can be deployed anywhere in the world.

Typical Applications
- Healthcare facilities
- Pharmaceutical
- Medical products
- Semiconductor & electronics manufacturing
- Food processing
- Agriculture
- Packaging
- Research laboratories

Cost-effective Solution
The continuous monitoring approach can deliver significant value in return for minimal investment. Automation saves time and resource taken up by manual sampling processes while early warnings can minimise costly downtime or other significant costs associated with undetected contamination.
The cost model is simple and comprises:
- Purchase cost for each monitoring device includes secure data hosting, software and integrations
- No subscription charges
- Single gateway can be used for up to 50 devices
- Options for sensor calibration service
Preparing for Annex 1
- Continuous monitoring of particles + environmental conditions
- Particle size categorisation (24 particle bins)
- Automated real-time alerts giving early warning of drift from normal operating conditions
- Repository of granular data to support trend analysis and threat assessment
Partner with the Sensor Experts

NuWave Sensors was established in 2014 with the R&D team based in Ireland together with customer support and commercial teams across Ireland, UK and US.
NuWave collaborates with commercial reseller partners to unlock value and deliver scalable solutions for customers around the world.
- Flexible partner program with enterprise options
- All products manufactured in Ireland to the highest QA standards
- Established supply chain resilience and consistent delivery lead-times of 3-6 weeks
- Dedicated onboarding and support team